“deltaFLUUniversal Influenza Vaccine
For Broad Protection and Superior Efficacy
”
Vivaldi Biosciences is developing its deltaFLU universal influenza vaccine to protect against all influenza strains – seasonal and pandemic – for all ages, from infants to seniors.
deltaFLU is an advanced, self-adjuvanting influenza vaccine, administered as a nasal spray. Immunization with deltaFLU is simple, convenient and pain-free.
Universal Protection
Read more...
Innovative Production
Read more...
Clinical Trials
Read more...
“The
deltaFLU Difference”
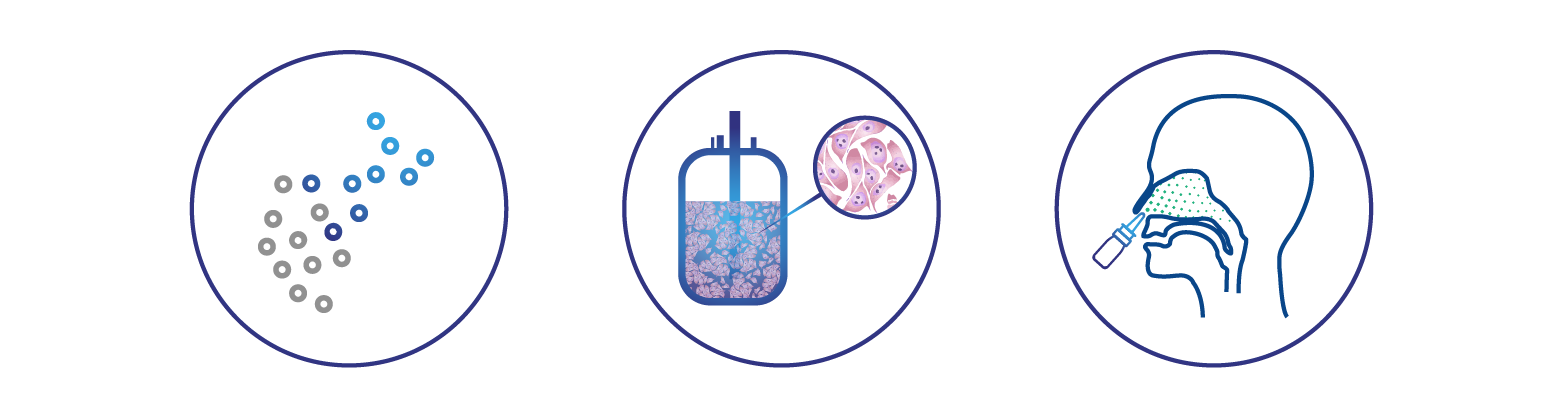
deltaFLU is the only vaccine in clinical development based on deletion of the influenza NS1 gene. Removing this influenza virulence gene gives deltaFLU both its unique mode of attenuation and its highly effective mechanism of action. Lacking NS1, deltaFLU rapidly induces interferon, a key component of the immune response to viral infection.
Deletion of NS1 achieves two highly advantageous properties of deltaFLU:
Delivered into the nasal passages in a gentle spray, deltaFLU introduces to the immune system natural viral antigens that stimulate multiple components of the immune system to achieve a broadly protective immune response. The effect is similar to infection with a circulating influenza strain, but with a crucial difference: deltaFLU vaccine strains are replication-deficient. Because of this, deltaFLU strains cannot cause disease and do not produce viral progeny. Individuals immunized with deltaFLU do not shed the vaccine virus. These are important safety aspects of the deltaFLU approach, and significant advantages over live vaccines that are able to replicate.
deltaFLU vaccine strains can’t fight off the body’s interferon response because unlike naturally occurring influenza viruses, they lack the ability to produce NS1. As a result, deltaFLU rapidly induces high levels of interferon and IgA antibodies in the linings of the nasal passages, creating a first line of defense against circulating disease-causing influenza viruses directly at their point of entry. This potent interferon response generates a self-adjuvant effect that activates a broadly protective systemic immune response in the form of serum antibodies, T cells, and T cell-based memory to viral infection.
Rapid and robust interferon induction – and interferon’s self-adjuvant effect – are unique to deltaFLU.